Hydrophobic Coatings
What is a hydrophobic coating?
Definition
“Phobic” comes from the Greek root “phobos,” meaning “fear”, and “hydro” refers to water. It follows that the literal meaning of hydrophobic is “afraid of water.” The term hydrophobic commonly refers to a surface that repels water and water-based materials.
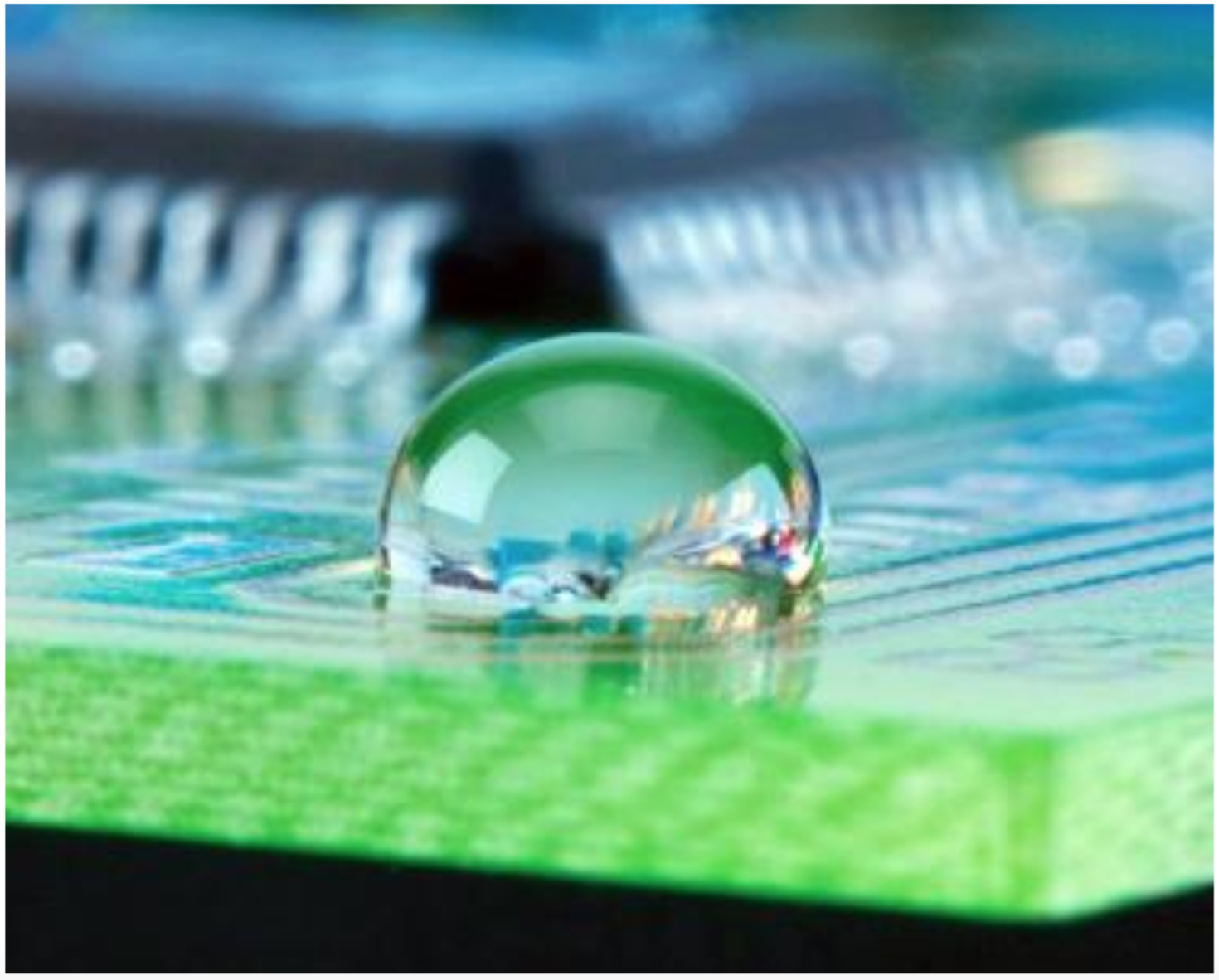
When water and water-based materials come in contact with a hydrophobic surface, individual drops of will bead up and will roll off or slide off the surface with ease. Larger amounts of water will pool together, the edges pulling back from the hydrophobic surface and move as one sheet.
This effect can also be described as dewetting, where the water pulls away leaving the surface totally dry.
A classic example is the leaf of the lotus plant. The surface of the leaves is so repellent to water that the surface is said to be “superhydrophobic.” Water seems to form near perfect spheres when coming in contact with the leaf surface.
With the potential to impart properties such as stay-dry, easy-to-clean, waterproof, anti-corrosion, and reduced-hydro-drag, hydrophobic coatings and treatments are finding their way into more mainstream applications.

How can the degree of hydrophobicity be measured?
This can be determined by measuring how “spherical” a water drop is as it sits on the hydrophobic surface. As the hydrophobicity of the surface increases, the high surface tension of the water pulls itself away from the surface more and more into a sphere.
If a line is drawn tangent to the drop as it contacts the surface, then the angle (θ) between that line, and a line parallel with the surface, is said to be the contact angle (CA). Surfaces are considered to be hydrophobic if the contact angle is between 80° and 120°.
An example of historic importance is polytetrafluoroethylene (Teflon) with a contact angle of 109°. Polytetrafluoroethylene is well known for its repellent, non-stick and non-friction surface. Surfaces with a contact angle from 120o to 180o are considered superhydrophobic.
Theoretically, the maximum hydrophobicity is 180° and in this case, a drop is perfectly spherical, contacting the surface at a singular, almost imperceptible point. Water and water-based materials will appear to bounce off a superhydrophobic surface. It seems the superhydrophobic surface is exerting a force against the water droplet and pushing it away, however that is not the case. There is simply no attraction between the surface and the water droplet.
If there was an attraction, the surface would be considered hydrophilic. This means “water-loving.” Hydrophilic surfaces attract water and allow it to level out or wet out. This property is used, for example, on the surface of self-cleaning windows. Instead of water forming discrete droplets, it forms sheets that quickly flow down and off, taking some of the dust and dirt off the window with it.
To measure the contact angle, an instrument called a goniometer is used. “Goni” is the Greek prefix for “angle.”

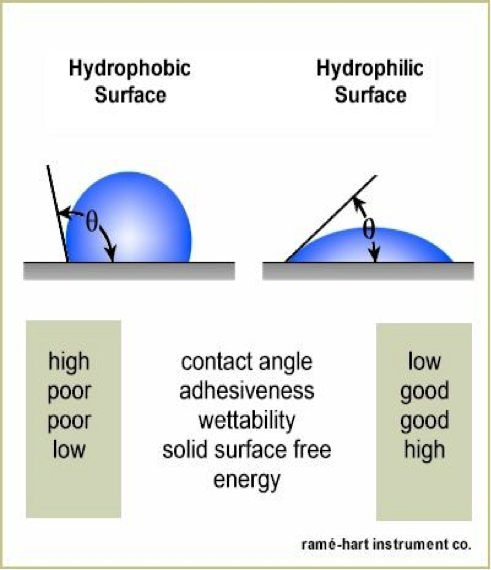
Hydrophilic: Surfaces with a contact angle of 10-80 degrees
Super-hydrophilic: Surfaces with a contact angle of 0-10 degrees
This instrument deposits a drop of liquid onto the surface of the substrate and then measures the angle using a video camera and specialized software. More sophisticated equipment and software programs enable the researcher to analyze the surface in different ways. In fact, surface science, or the study of surface chemistry and its properties, is expanding rapidly.
Nanoslic is a Hydrophobic Coating
NanoSlic coatings are hydrophobic with water contact angles between 105°-107°. These coatings achieve this high degree of water repellency through a chemical reaction that takes place during the curing phase. As the solvent dries, highly non-polar molecules align themselves and form the coating’s surface. Below this layer, the bulk of the coating polymerizes into an inert silica-based structure. At the substrate surface, the polymer chemically bonds to hydroxyl groups on the substrate. The advantage of this approach is that a high degree of adhesion and abrasion resistance is achieved. Therefore, NanoSlic coatings are an excellent choice for the most demanding applications and challenging environments.
