Waterproof Coating
Water has often been called the “universal solvent.” That is because it dissolves more substances than any other liquid. This fact is important to every living thing on earth and cannot be overstated.
It means that wherever water goes, either through the ground or through our bodies, it takes along valuable chemicals, minerals, and nutrients. In this way, it functions as a transport medium for substances that living things require.
From another perspective, when water comes in contact with a surface, it often a reacts chemically and physically, to negatively impact the surface, even destroy it.
Water is the Ultimate Solvent
It is water’s unique chemical composition and physical attributes that make it such an excellent solvent.
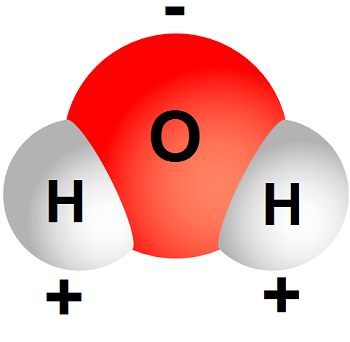
Water molecules have a polar arrangement of the oxygen and hydrogen atoms—one side (hydrogen) has a positive electrical charge and the other side (oxygen) had a negative charge.
This allows the water molecule to become attracted to many other different types of molecules.
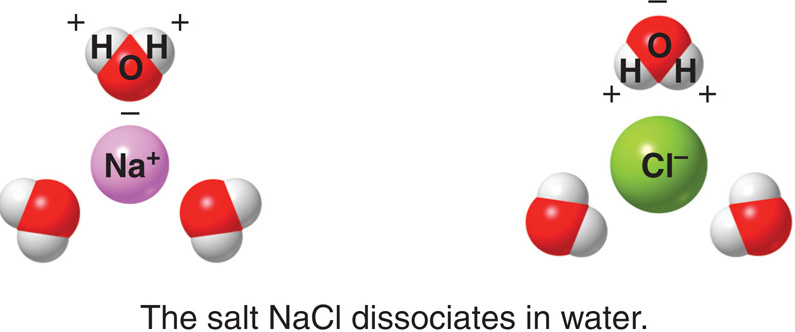
Water can become so heavily attracted to a different molecule, like common salt (NaCl), that it can disrupt the attractive forces that hold the sodium and chloride in the salt molecule together and, thus, dissolve it.
At the molecular level, salt dissolves in water due to electrical charges and due to the fact that both water and salt compounds are polar, with positive and negative charges on opposite sides of the molecule.
The bonds in salt compounds are called ionic because they both have an electrical charge—the chloride ion is negatively charged and the sodium ion is positively charged.
Likewise, a water molecule is ionic in nature, but the bond is called covalent, with two hydrogen atoms both situating themselves with their positive charge on one side of the oxygen atom, which has a negative charge.
When salt is mixed with water, the salt dissolves because the covalent bonds of water are stronger than the ionic bonds in the salt molecules.
Chemical Reactions and Corrosion
Water causes chemical reactions when it comes into contact with other materials, for example, metals. This effect of the universal solvent negatively affects structures worldwide.
The most common result is rust, which is the common name for iron oxide.
Iron oxide, the chemical Fe2O3, is so common because iron combines very readily with oxygen.
Iron (or steel) rusting is an example of a water-induced effect called corrosion — an electrochemical process involving an anode (a piece of metal that readily gives up electrons), an electrolyte (a liquid that helps electrons move) and a cathode (a piece of metal that readily accepts electrons).

When a piece of metal corrodes, the electrolyte helps provide oxygen to the anode.
As oxygen combines with the metal, electrons are liberated.
When these electrons flow through the electrolyte to the cathode, the metal of the anode disappears, swept away by the electrical flow or converted into metal cations in a form such as rust.
For iron to become iron oxide, three things are required: iron, water, and oxygen. Here’s what happens when the three get together:
When a drop of water hits an iron object, two things begin to happen almost immediately.
- First, the water, a good electrolyte combines with carbon dioxide in the air to form a weak carbonic acid, an even better electrolyte.
- Second, as the acid is formed and the iron dissolved, some of the water will begin to break down into its component pieces — hydrogen and oxygen.
- Third, the free oxygen and dissolved iron bond into iron oxide, in the process freeing electrons. The electrons liberated from the anode portion of the iron flow to the cathode, which may be a piece of a metal less electrically reactive than iron, or another point on the piece of iron itself.
The chemical compounds found in liquids like acid rain, seawater and the salt-loaded spray from snow-belt roads make them better electrolytes than pure water, allowing their presence to speed the process of rusting on iron and other forms of corrosion on other metals.

Water also negatively impacts other surfaces by erosion, staining and its ability to deposit foreign materials.
To protect these materials and surfaces from the universal solvent, “waterproof coatings” are often used.
Generally, the term “waterproof coating” is broadly used and can imply any water impermeable barrier between a surface and water.
By this definition, common architectural paints and coatings comprised of alkyd, acrylic, urethane, and epoxy polymers are said to “waterproof” a surface because they provide a physical barrier.
Nanoslic Provides a Spray-On/Wipe-On Water Proofing
NanoSlic technology goes far beyond a physical barrier and provides a hydrophobic surface that chemically interacts with water to repel it.
Water beads up, pools together and slides off the surface to provide real, long-term protection. Universal solvent meets universal protection.
